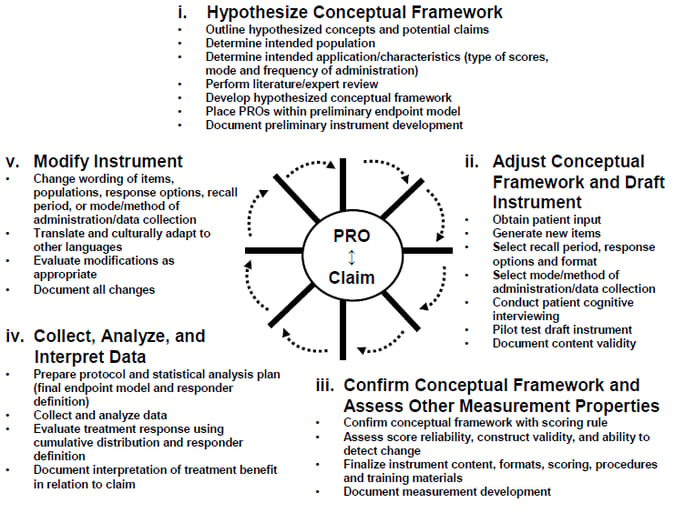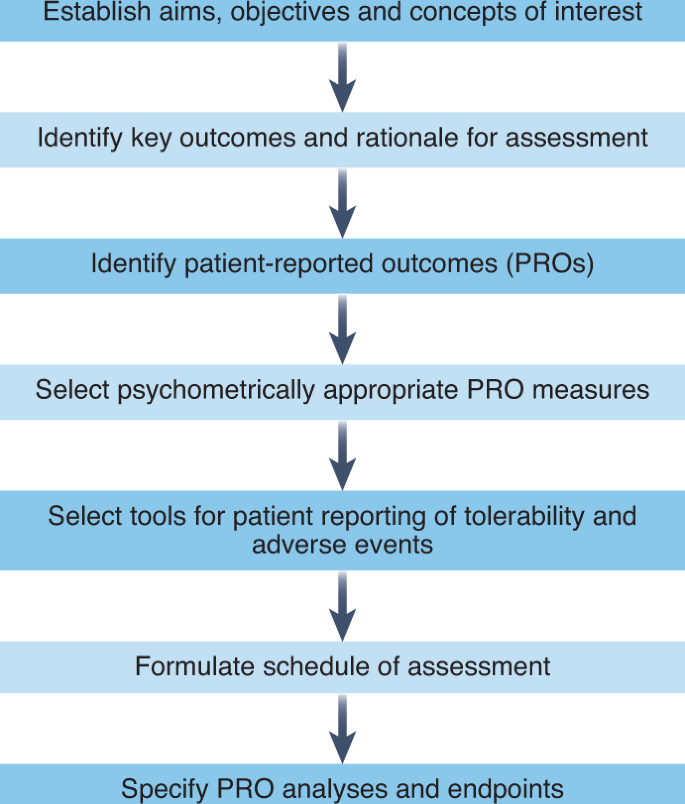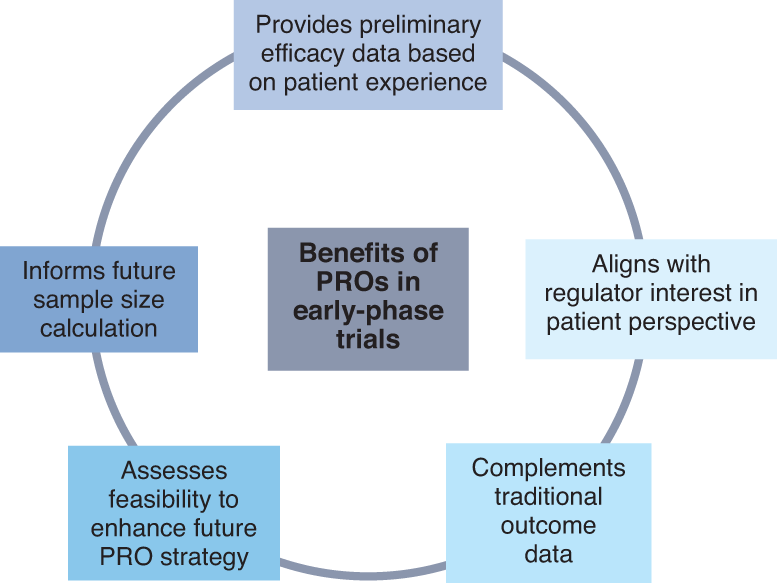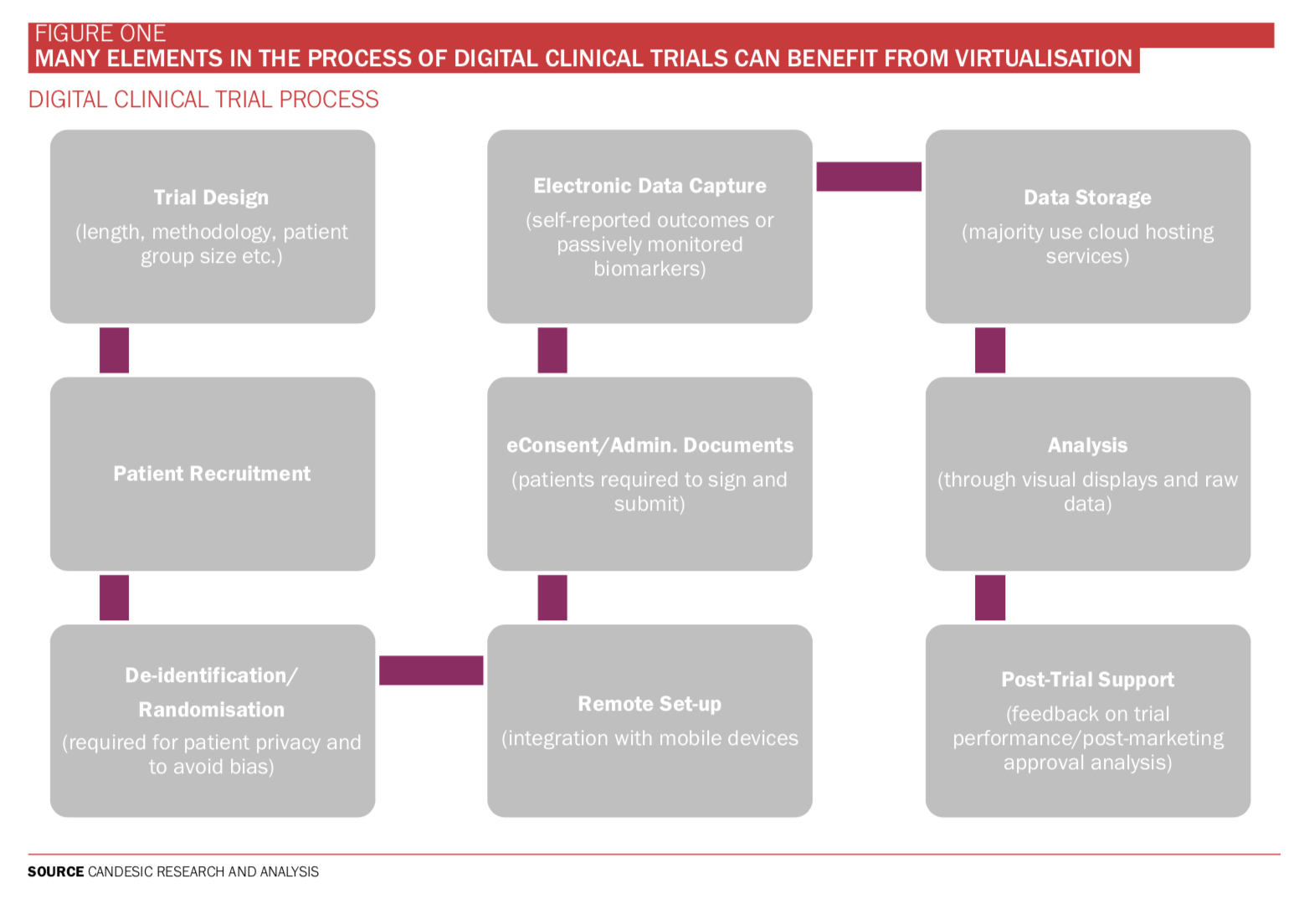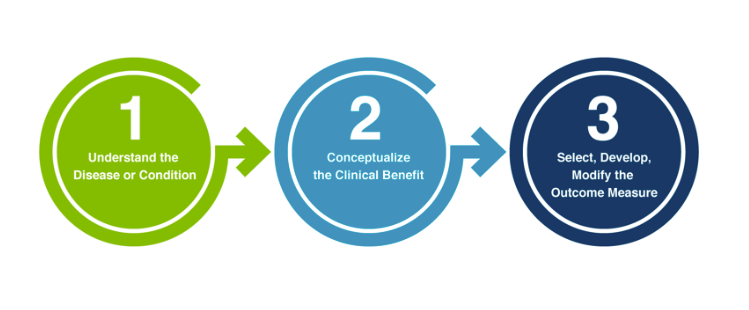
FDA Patient-Focused Drug Development Guidance Update: Incorporating Patient Experience Data in Clinical Trials
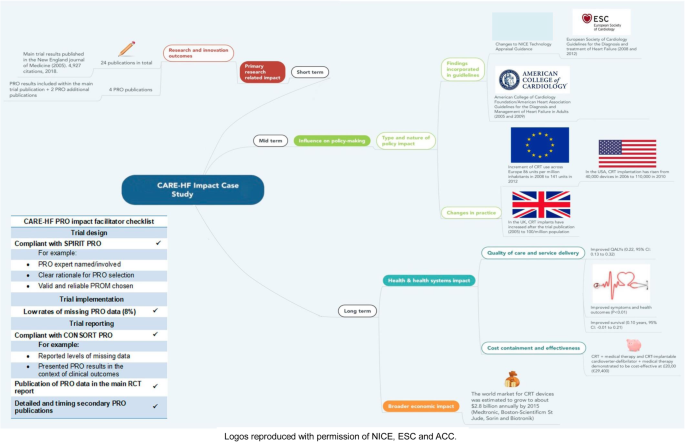
The impact of patient-reported outcome (PRO) data from clinical trials: a systematic review and critical analysis | Health and Quality of Life Outcomes | Full Text

Patient-reported outcomes: A new era in clinical research Deshpande PR, Rajan S, Sudeepthi B L, Abdul Nazir C P - Perspect Clin Res

OutcomesFacilitator (PROMs) on Twitter: "Patient reported measures such as Patient Reported Outcome Measures (PROMs) and Patient Reported Experience Measures (PREMs) should become common currency in the assessment of the benefits and risks
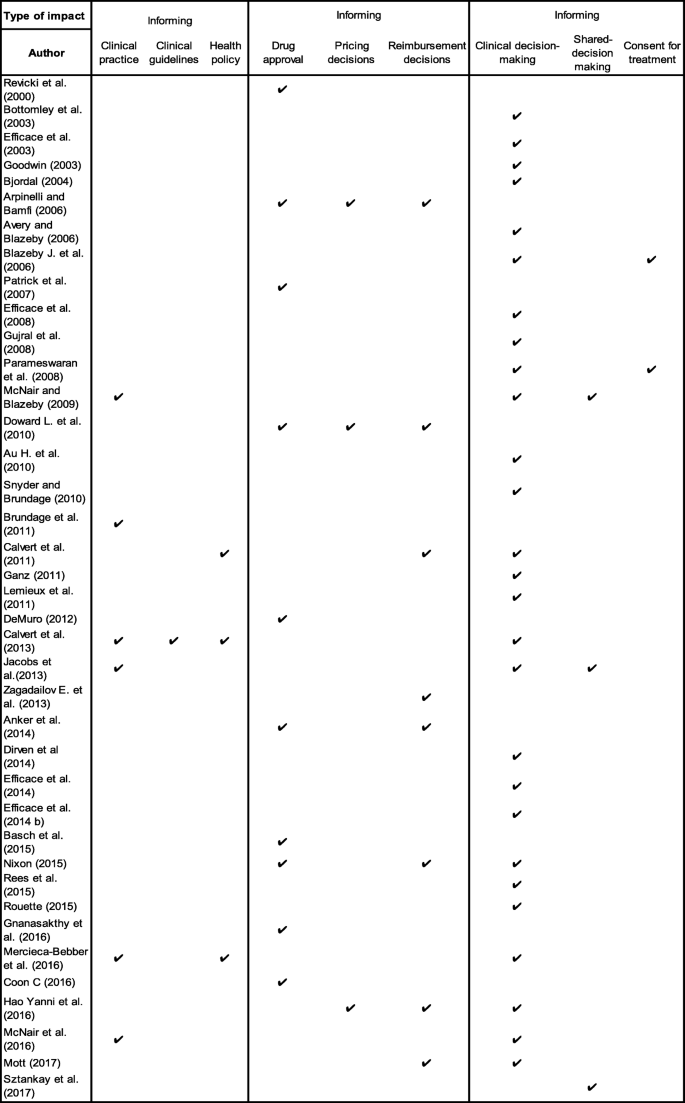
The impact of patient-reported outcome (PRO) data from clinical trials: a systematic review and critical analysis | Health and Quality of Life Outcomes | Full Text
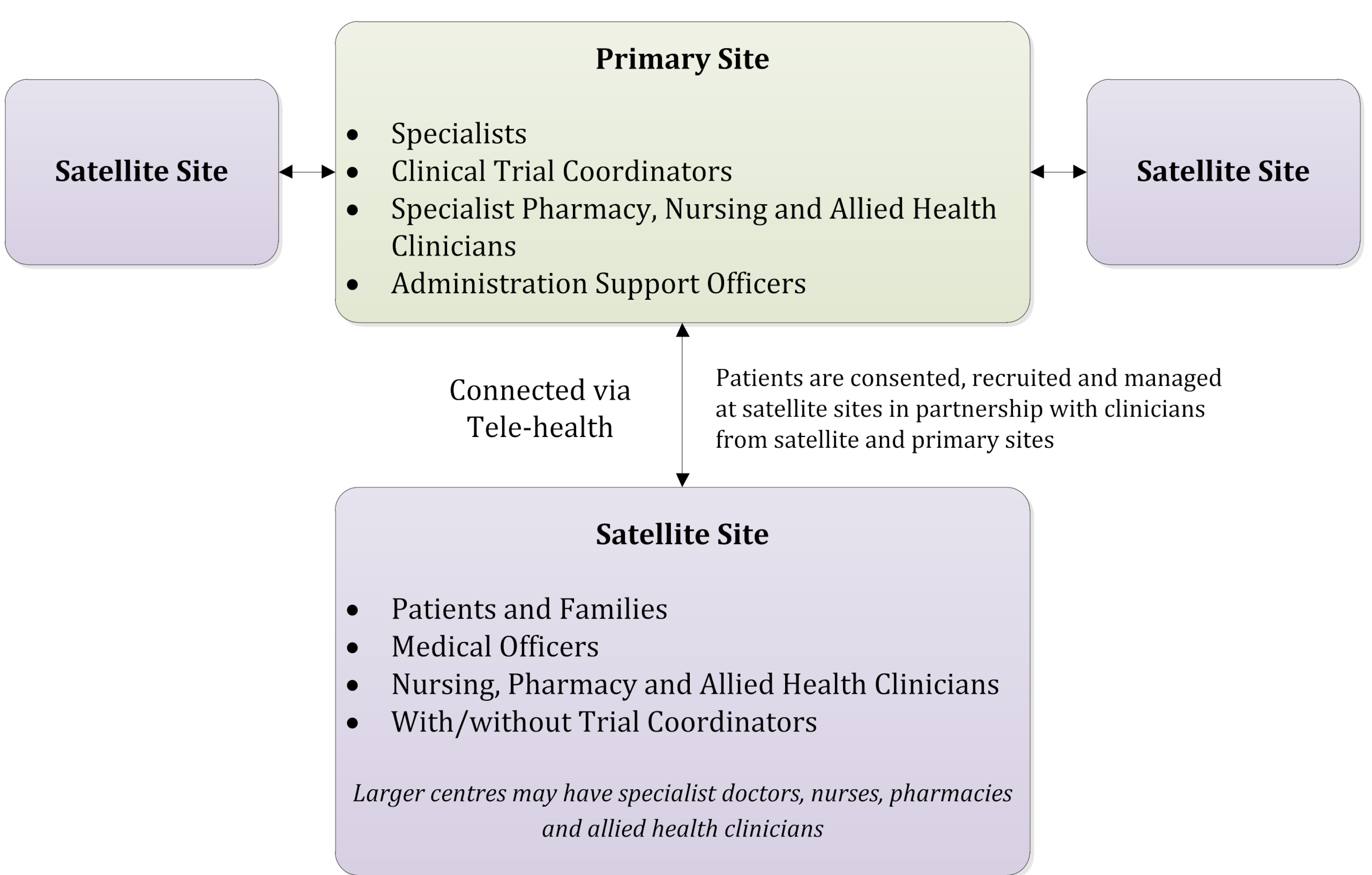
Current Oncology | Free Full-Text | CRAFT—A Proposed Framework for Decentralized Clinical Trials Participation in Canada

Analysing data from patient-reported outcome and quality of life endpoints for cancer clinical trials: a start in setting international standards - ScienceDirect

Cardiovascular outcomes in adults with hypertension with evening versus morning dosing of usual antihypertensives in the UK (TIME study): a prospective, randomised, open-label, blinded-endpoint clinical trial - The Lancet